Abstract
Background. Polycythemia vera (PV) patients are treated with periodic therapeutic phlebotomy (TP) alone or in combination with either hydroxyurea (HU), ruxolitinib (RUX) or interferon (IFN) to maintain hematocrit (HCT) levels below 45% as per NCCN guidelines. Since patients are seen periodically, PV patients may spend significant time with HCT levels above 45%, thereby increasing their risk of thrombosis [Marchioli NEJM 2013]. PV is associated with systemic symptoms with fatigue. These fatigue-related symptoms are found to be the most prevalent and severe as reported in an international survey among PV patients [Scherber Cancer 2016]. Symptomatic iron deficiency represents an unaddressed clinical challenge to PV patients as most PV patients have iron deficiency at diagnosis due to increased iron utilization [Ginzburg Leukemia 2018]. The iron deficiency worsens after repeated TP. We have demonstrated in a phase 2 study that rusfertide (PTG-300) has a good tolerability profile and achieves HCT control in PV patients with improvement in iron deficiency.
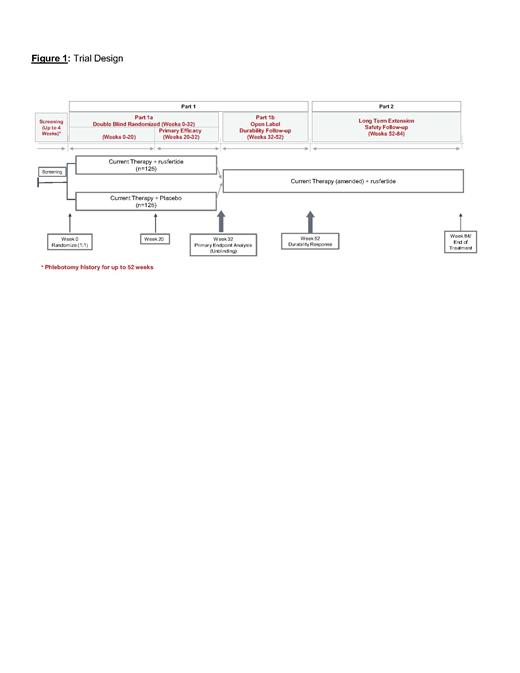
Methods. This is a Phase 3, multicenter, global, randomized trial that compares the efficacy and safety of rusfertide compared to placebo when added on to current therapy for PV (Figure 1). The study population is PV subjects who require frequent phlebotomies to control their hematocrit with or without concurrent therapy. This is a three-part study in subjects with polycythemia vera:
- Part 1a: randomized, double-blind, placebo-controlled, add-on parallel-group period for 32 weeks. Subjects will be stratified by their ongoing PV treatment and randomized 1:1 to rusfertide or placebo added-on to their ongoing PV treatment.
- Part 1b: open-label treatment phase during which all subjects who complete Part 1a successfully will receive rusfertide for 20 weeks (Week 32 through Week 52).
- Part 2: Long term extension (LTE) phase during which all subjects who complete Part 1b will continue to receive rusfertide for 32 weeks (Week 52 to Week 84).
Inclusion Criteria: Approximately 250 subjects will be randomized. Eligibility criteria include PV diagnosis (by 2016 WHO criteria) and frequent phlebotomies with or without concurrent cytoreductive therapy to maintain HCT below 45% in the 6 months prior to enrollment in Part 1. Eligible subjects will continue to receive their therapy at screening for PV (phlebotomy alone (TP) or cytoreductive therapy + TP) and must have a hematocrit <45% at Week 0 prior to randomization. Subjects who meet the eligibility criteria will be stratified by ongoing PV therapy (TP only, TP+hydroxyurea, TP+ruxolitinib, TP+interferon, TP+other) and randomized 1:1 to treatment with rusfertide or placebo added on to the subject's ongoing PV therapy at Week 0.
The "add on" design allows subjects to receive standard cytoreductive therapy to control WBC and/or platelets and to receive rusfertide/placebo. The dose of cytoreductive therapy in Part 1a and Part 1b may be decreased for safety but may not be increased for efficacy including control of hematocrit, elevated platelets and/or WBC.
Primary endpoint:
Proportion of subjects achieving a response starting at Week 20 through Week 32 (inclusive) who receive rusfertide compared to placebo. A response is defined as absence of phlebotomy eligibility defined as either:
1. a confirmed hematocrit ≥45% and that is at least 3% higher than the baseline hematocrit (value immediately prior to randomization at Week 0); confirmation required within 1 to 7 days, or
2. a hematocrit ≥48%.
Key words: Hepcidin, Hematocrit, Rusfertide, PTG-300, Polycythemia Vera, PV, Therapeutic Phlebotomy
Verstovsek: Incyte Corporation: Consultancy, Research Funding; Gilead: Research Funding; PharmaEssentia: Research Funding; Protagonist Therapeutics: Research Funding; CTI BioPharma: Research Funding; Ital Pharma: Research Funding; NS Pharma: Research Funding; Roche: Research Funding; Genentech: Research Funding; Blueprint Medicines Corp: Research Funding; Celgene: Consultancy, Research Funding; Promedior: Research Funding; AstraZeneca: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Kuykendall: Pharmaessentia: Honoraria; Protagonist: Consultancy, Research Funding; Novartis: Honoraria, Speakers Bureau; Celgene/BMS: Honoraria; Abbvie: Honoraria; Incyte: Consultancy; Blueprint: Honoraria. Hoffman: AbbVie Inc.: Other: Data Safety Monitoring Board, Research Funding; Novartis: Other: Data Safety Monitoring Board, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Kartos Therapeutics, Inc.: Research Funding. Pemmaraju: Incyte: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; MustangBio: Consultancy, Other; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Celgene Corporation: Consultancy; DAVA Oncology: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Protagonist Therapeutics, Inc.: Consultancy; Roche Diagnostics: Consultancy; LFB Biotechnologies: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Plexxicon: Other, Research Funding; Samus: Other, Research Funding; Sager Strong Foundation: Other; Aptitude Health: Consultancy; Affymetrix: Consultancy, Research Funding; CareDx, Inc.: Consultancy; Springer Science + Business Media: Other; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Cellectis S.A. ADR: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Valone: Protagonist Therapeutics: Consultancy, Current equity holder in publicly-traded company. Modi: Protagonist Therapeutics: Current Employment. Khanna: Protagonist: Current Employment, Current equity holder in publicly-traded company. O'Connor: Protagonist Therapeutics: Current Employment. Gupta: Protagonist Therapeutics: Current Employment. Kiladjian: Taiho Oncology, Inc.: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Other: Personal fees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal